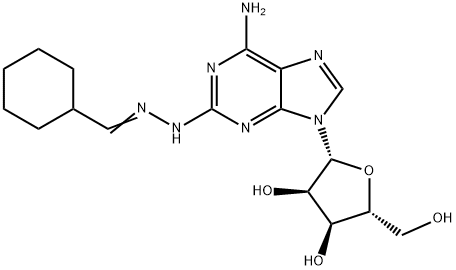
Binodenoson, 2-[(cyclohexylmethylene)hydrazino] adenosine (MRE0470), is being developed as a short-acting coronary vasodilator, administered intravenously, as an adjunct to radiotracers in myocardial stress imaging techniques to detect the presence and severity of CAD in patients unable to exercise. The compound is a highly selective agonist at the adenosine A2A receptor and also has a weaker affinity for adenosine A1-, A2B-, and A3-receptor subtypes than adenosine[1].
2-(cyclohexylmethylidenehydrazino)adenosine is used in the diagnosis of coronary heart disease (adenosine A2A agonist).
The mean values for the apparent elimination half-life of binodenoson ranged from 7.4 minutes (at 1 g/kg) to 14.9 minutes (6 g/kg), with a slight tendency toward higher values with increasing doses. However, because the plasma concentrations for the lowest dose level (0.4 g/kg) were only marginally higher than the assay's lower limit of quantitation, the plasma concentrations could be determined for a longer period of time at the higher dose levels. On average (harmonic mean), the terminal half-life of binodenoson across all doses was 10±4 minutes[1].
Binodenoson (MRE-0470) (30-300 nM) decreases oxidative activity of tumor necrosis factor-α–primed FMLP-stimulated polymorphonuclear leukocytes in human whole blood and acts synergistically with Rolipram.
Binodenoson (infused 0-0.9 μg/kg/h; adult Wistar rat; rat bacterial meningitis model), with or without rolipram (0-0.01 μg/kg/h), inhibits pleocytosis and reduces the lipopolysaccharide-induced increase in blood-brain barrier permeability (BBBP), indicative of decreased neutrophil-induced damage.
[1] Richard J. Barrett PhD . “Pharmacokinetics and safety of binodenoson after intravenous dose escalation in healthy volunteers.” Journal of Nuclear Cardiology 12 2 (2005): Pages 166-171.