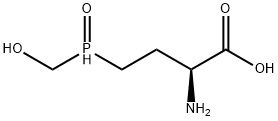
A 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid that has S configuration at position 2. A glutamine synthetase inhibitor, it is used (generally as the corresponding ammonium or sodium salts, known as glufosinate-P-ammonium and glufosinate-P-sodium, respectively) as a herbicide to control annual weeds and grasses.
ChEBI: Glufosinate-P is a 2-amino-4-[hydroxy(methyl)phosphoryl]butanoic acid that has S configuration at position 2. A glutamine synthetase inhibitor, it is used (generally as the corresponding ammonium or sodium salts, known as glufosinate-P-ammonium and glufosinate-P-sodium, respectively) as a herbicide to control annual weeds and grasses. It has a role as a herbicide, an EC 6.3.1.2 (glutamate--ammonia ligase) inhibitor and an agrochemical. It is an enantiomer of a (2R)-glufosinate. It is a tautomer of a glufosinate-P zwitterion.
Through oxidative stress C. elegans, racemic glufosinate-ammonium l-glufosinate-ammonium (Glufosinate-P) both mediated developmental toxicity (shortened developmental cycle, reduced body length width, promoted aging and decreased longevity), neurotoxicity (inhibited head swinging, body bending frequency acetylcholinesterase [AchE] activity) reproductive toxicity (significant reductions in number eggs offspring in vivo induced apoptosis gonadal cells). The degradation rate of L-glufosinate-ammonium was faster than that of D-glufosinate-ammonium. Based on MDA content, protein content, and antioxidant enzyme (SOD CAT) activity, Meng et al. found that L-glufosinate-ammonium could cause more severe oxidative damage than rac-glufosinate-ammonium[1-2].
[1] Xu Zhao . “Comparison of the chronic and multigenerational toxicity of racemic glufosinate and l-glufosinate to Caenorhabditis elegans at environmental concentrations.” Chemosphere 316 (2023): Article 137863.
[2] Tianyou Feng. “Comparative analysis of toxicity and metabolomic profiling of rac-glufosinate and L-glufosinate in zebrafish.” Aquatic Toxicology 261 (2023): Article 106618.