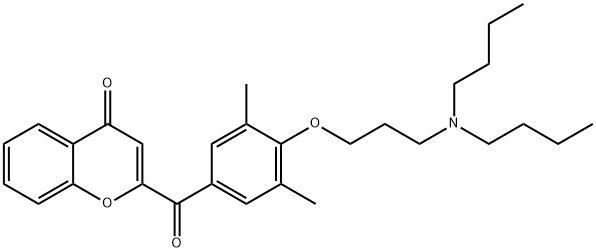
Bucromarone
- Product NameBucromarone
- CAS78371-66-1
- MFC29H37NO4
- MW463.61
- EINECS
- MOL File78371-66-1.mol
Usage And Synthesis
1.2 mol of an electrophilic catalyst, advantageously aluminum chloride, was
slowly added to a solution of 48.8 g (0.4 mol) of 2,6-dimethyl phenol in 400
ml of dichloroethane and agitated at room temperature for an hour. The
mixture was cooled to 0°C and a solution of 84.0 g (0.4 mol) of 2-carboxylic
chromone acid chloride in 400 ml of a dichloroethane, was slowly added.
Agitation was continued at 0°C for 5 h and then at room temperature for 4
days. The reaction mixture was poured into 1.6 L of iced 50% hydrochloric
acid. The resulting precipitate was filtered, washed with water, dried and
106.0 g (72% yield) of 2-(3,5-dimethyl-4-hydroxybenzoyl)chromone, melting
point 216°C (recrystallised from dioxane) were obtained.
6.9 g (0.05 mol) of potassium carbonate was added to a solution of 29.4 g (0.1 mol) of 2-(3,5-dimethyl-4-hydroxybenzoyl)chromone in 250 ml of dimethyl formamide and kept at 100°C for an hour. A solution of 20.5 g (0.1 mol) of 3-N,N-dibutylamino-1-chloropropane in 100 ml of diethyl formamide was then added and the resulting solution heated to 130°C for 3 h. The solution was filtered, the dimethyl formamide was concentrated, dissolved in 300 ml water and extracted twice with 200 ml of benzene. The organic phase was dried on magnesium sulfate and the hydrochloride was precipitated by adding hydrochloric ether. 40.0 g (yield 80%) of 2-[4-(3-N,N-dibutylaminopropoxy)- 3,5-dimethylbenzoyl]chromone were obtained (recrystallised from an acetone/ether mixture).
6.9 g (0.05 mol) of potassium carbonate was added to a solution of 29.4 g (0.1 mol) of 2-(3,5-dimethyl-4-hydroxybenzoyl)chromone in 250 ml of dimethyl formamide and kept at 100°C for an hour. A solution of 20.5 g (0.1 mol) of 3-N,N-dibutylamino-1-chloropropane in 100 ml of diethyl formamide was then added and the resulting solution heated to 130°C for 3 h. The solution was filtered, the dimethyl formamide was concentrated, dissolved in 300 ml water and extracted twice with 200 ml of benzene. The organic phase was dried on magnesium sulfate and the hydrochloride was precipitated by adding hydrochloric ether. 40.0 g (yield 80%) of 2-[4-(3-N,N-dibutylaminopropoxy)- 3,5-dimethylbenzoyl]chromone were obtained (recrystallised from an acetone/ether mixture).
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine