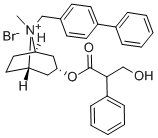
2 methods of preparation of endo-(+/-)-8-([1,1'-biphenyl]-4-ylmethyl)-3-(3-
hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-azoniabicyclo[3.2.1]octane
bromide.
1). 28.9 g of dl-tropic acid ester of the tropine is dissolved in 300 ml of luke-
warm acetone and to the solution are added 25.0 g of 4-diphenyl-methyl-
bromide, dissolved in 75 ml of acetone. The solution is kept for 1 h at room
temperature, thereafter during 6 h at 40-60°C. The separated quaternary salt
is filtered off, washed with acetone and dried at gentle heat. The endo-(+/-)-
8-([1,1'-biphenyl]-4-ylmethyl)-3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-
methyl-8-azoniabicyclo[3.2.1]octane as snow-white microcrystalline powder,
melting point 220-222°C (dec., recystallizing from ethyl alcohol) is obtained
(yield 35.0-39.0 g).
2). 14.1 g of trans-tropanol are dissolved in 150 ml of acetone and the
solution mixed with 24.7 g of 4-diphenyl-methyl-bromide dissolved in a little
acetone. The solution is kept for some hours at about 50°C and therefater the
separated 4-diphenyl-methyl-trans-tropinium-bromide is isolated in the usual
manner. Yield about 90%. After recrystallising in ethyl alcohol the compound
melts at about 230°C (dec.).
19.4 g of the quarternary aminoalcohol obtained in the above manner is
mixed with 13.5 g of O-acetyl-dl-tropic acid bromide, in a vessel provided with
a tube containing calcium chloride, and the mixture is warmed in an oil bath
at 120-130°C until no more development of hydrogen bromide gas (approx. 3
h) takes place. For the purpose of splitting of the acetyl-residue the resulting
ester is boiled for 0.5 h with 50 ml of 10% hydrobromic acid and the resulting
solution evaporated to dryness in vacuo. On the endo-(+/-)-8-([1,1'-
biphenyl]-4-ylmethyl)-3-(3-hydroxy-1-oxo-2-phenylpropoxy)-8-methyl-8-
azoniabicyclo[3.2.1]octane, melting point 221°C (dec., recrystallization from
alcohol) is obtained.
Poison by intravenous, subcutaneous, and intraperitoneal routes. Moderately toxic by ingestion. Experimental reproductive effects. When heated to decomposition it emits very toxic fumes of NOx and Br-. See also BROMIDES.