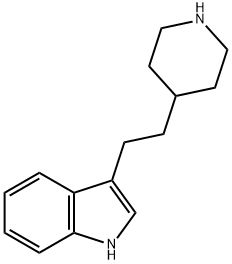
Indalpine is a non-tricyclic antidepressant with a serotonin selective profile. It is
6-7 times more potent than fluoxetine and clomipramine in inhibiting serotonin
reuptake in vitro in rat brain synaptosomes. Statistically significant clinical
effects within one week of onset of treatment have been reported. An anxiolytic
effect may accompany the antidepressant effect. Indalpine appears devoid of
anticholinergic and cardiovascular side effects and does not promote weight gain
or affect appetite.
ChEBI: Indalpine is a member of indoles.
0.5 g of bis(methoxy-2ethoxy)sodium aluminum hydride in a 70% solution in
toluene is added to a solution of 0.29 g of (indolyl-3)(piperidyl-4-methyl)
ketone in 10 ml of toluene. The mixture is heated under refluxing conditions
for 15 hours, then cooled to 0°C. 10 ml of an aqueous solution of 5N sodium
hydroxide is added dropwise thereto, followed by stirring for 1 hour. The
organic phase is decanted, washed with water, dried using potassium
carbonate and evaporated under partial vacuum. 0.26 g of oil is obtained,
which is purified by chromatography and hydrochloride formation. The productobtained is 0.1 g of (indolyl-3)-2-ethyl-4-piperidine hydrochloride which has a
melting point of 167°C.
World Health Organization (WHO)
Indalpine, an antidepressant with serotoninergic action, was
introduced in 1983 and marketed exclusively in France. In 1984 its use was
associated with cases of leucopenia and agranulocytosis which led to the
voluntary suspension of clinical trials in the USA. In 1985 the major manufacturer
voluntarily withdrew the drug from the market.