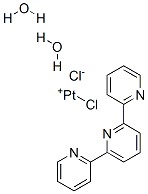
Recrystallise it from hot dilute HCl, and on cooling it gives the red dihydrate. The trihydrate crystallises slowly from a cold aqueous solution and is dried in air. The red dihydrate can be obtained from the trihydrate by desiccation over conc H2SO4, by washing with EtOH or by precipitating from a warm aqueous solution with HCl. The dihydrate is also formed by decomposing the black trihydrate form by heating in water (slowly), or more rapidly with hot 2N HCl. [Morgan & Burstall J Chem Soc 1498 1934, Lippard Acc Chem Res 11 211 1987.]