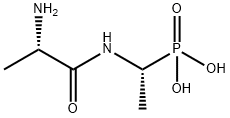
14.1 g (0.168 mol) of solid sodium bicarbonate were added to a solution of 7
g (0.056 mol) of (1R,S)-1-aminoethylphosphonic acid in 280 ml of water and
140 ml of ethanol while stirring at 0°C. While stirring this mixture at 0°C, a
solution of 17.9 g (0.056 mol) of the N-hydroxysuccinimide ester of N-benzyloxycarbonyl-L-alanine in 140 ml of warm ethanol was added dropwise
over ca 15 minutes. The latter solution was washed in with 70 ml of ethanol.
The heterogeneous mixture was stirred for 1 hour at 0°C and then for a
further 16 hours at room temperature, the mixture becoming homogeneous.
The mixture was evaporated and re-evaporated with 200 ml of water to give a
gum, which was dissolved in 500 ml of water. The solution was extracted
firstly with 500 ml of chloroform and then with 250 ml portions of chloroform,
acidified to pH 2 with ca 80 ml of 2 N hydrochloric acid and again extracted
with 500 ml of chloroform followed by two 250 ml portions of chloroform. The
aqueous layer was concentrated and passed down a column of cation
exchange resin (B.D.H., Zerolit 225, SRC 13, RSO3H; 750 g; freshly
regenerated in the acid cycle). The column was eluted with water and there
were collected six 250 ml fractions. The first four fractions were combined,
evaporated and re-evaporated with water to remove hydrogen chloride. There
was obtained a final residue of (1R,S)-1-[(N-benzyloxycarbonyl-L-alanyl)-
amino]ethylphosphonic acid which was separated as follows:
The latter residue was dissolved in 400 ml of water and titrated with 1 M
benzylamine to pH 4.5. The resulting solution was concentrated and
crystallized from water to give 5.3 g of the benzylamine salt of (1S)-1-[(Nbenzyloxycarbonyl-
L-alanyl)amino]ethylphosphonic acid of melting point 210-
215°C. Concentration of the mother liquors followed by further
recrystallization from water gave the benzylamine salt of (1R)-1-[(Nbenzyloxycarbonyl-
L-alanyl)amino]ethylphosphonic acid in a first crop of 0.59
g [melting point 226-228°C (decomposition); [α]D
20 = -32.3° (c = 1% in
acetic acid)] and a second crop of 0.825 g] melting point 225-227°C
(decomposition); [α]D
20 = -33.0° (c = 1% in acetic acid)]. Recrystallization of
the first crop from water gave 0.333 g of pure benzylamine salt of the Rstereoisomer;
melting point 226-228°C (decomposition); [α]D
20 = -33.1°
(c=1% in acetic acid).
1.1 g (2.5 mmol) of the benzylamine salt of (1R)-1-[(N-benzyloxycarbonyl-Lalanyl)
amino]-ethylphosphonic acid were dissolved in 4 ml of 2 N ammonium
hydroxide, passed down a column of cation exchange resin (B.D.H., Zerolit
225, SRC 13, RSO3H; 120 g; freshly regenerated in the acid cycle) and eluted
with water. There were collected 200 ml of acid eluate, which was
concentrated to 100 ml. To this were added 100 ml of methanol, 0.3 g of 5%
palladium-on-charcoal catalyst and 3 drops of glacial acetic acid. The mixture
was hydrogenated at room temperature and atmospheric pressure. The
catalyst was filtered off and the solvent evaporated. The residual gum was reevaporated
with three 50 ml portions of n-propanol to give 0.6 g of a gummy
solid of melting point ca 275-280°C (decomposition). After further
recrystallization from water and ethanol, there was obtained 0.2 g of (1R)-1-
(L-alanylamino)-ethylphosphonic acid of melting point 295-296°C
(decomposition); [α]D
20 = -44.0° (c=1% in water).
The different ways of synthesis of alafosfalin were described:
1). 1R-1-(L-alanylamino)-ethanephosphonous acid (0.034 M), mercuric
chloride (0.068 M) and water (175 ml) were mixed and heated to reflux for 1
hour The white insoluble mercuric chloride which formed was removed by
filtration and the aqueous filtrate was evaporated to dryness. The oily residue
was dissolved in ethanol (20 ml) and propylene oxide was added untilprecipitation was complete. Filtration gave 1R-1-(L-alanylamino)-
ethylphosphonic acid, which was recrystallised from ethanol/water. MP: 293-
295°C, [α]D
20 =-49.3° (1%, H2O). Yield was 100% of theory.
2). Papain (50 mg; 2.2 U/mg) was added to the solution of 0.5 mmol Z-L-Ala,
1.15 mmol racemic diisopropyl ester of 1-aminoethylphosphonic acid and 50
ml 2-mercaptoethanol in the mixture of 2.3 ml acetonitrile and 0.2 ml water
The suspension was shaken for about 2 days until all the Z-ala was consumed
(TLC-control). The enzyme was filtered off and washed with 10% KHSO4,
water, saturated NaHCO3, dried with anhydrous Na2SO4 and evaporated under
reduced pressure. The resulting phosphonopeptide was dissolved in 2 ml of
40% HBr in glacial acetic acid and left overnight. Anhydrous ether (10 ml)
was added and the mixture was stirred for 10 min and upper phase decanted.
The residue was evaporated, the remaining gum was dissolved in 2 ml of
methanol and treated with excess of propylene oxide. The precipitated
material was filtered off and crystallized from water water-ethanol to give
pure alafosfalin, yield 60%, MP: 273-276°C (decomposition); [α]D
20 =-45°
(0.2% in water). Only L-aminophosthonate is involved in the peptide bond
formation because of papain presence.