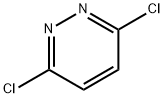
General procedure for the synthesis of 3,6-dimethoxypyridazine from methanol, 3,6-dichloropyridazine and sodium methanol: 3,6-dichloropyridazine (10.0 g, 67.12 mmol) and sodium methanol (9.79 g, 181.23 mmol) were dissolved in methanol (39 mL) and the reaction was heated at 70 °C overnight. Upon completion of the reaction, the mixture was cooled to room temperature, diluted with dichloromethane (200 mL) and subsequently washed with water (100 mL x 2). The organic phase was dried over anhydrous magnesium sulfate and concentrated to dryness under reduced pressure to afford the title compound 3,6-dimethoxypyridazine (9.46 g, 101% yield) as a white solid. The crude product could be directly used in the subsequent reaction without further purification. The structure of the product was confirmed by 1H NMR (300 MHz, CDCl3): δ 6.91 (s, 2H), 4.05 (s, 6H). Mass spectrometry (APCI) showed m/z = 182 ([M+H]+). HPLC analysis showed a retention time of 1.19 min.