plate-like crystal(s); available commercially as a water solution; readily forms hydrates; conc solution decomposes; used in baking powder(s) in the form of the sodium salt [HAW93]
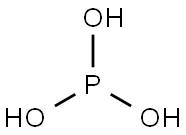
Hypophosphoric acid (H2PO3 or H4P2O6) is a solid, decomposing in solution to form phosphorous plus phosphoric acids. Hypophosphoric acid is used in solution and is a reducing agent, but only with strong oxidizing agents, such as potassium permanganate; and the acid is unaffected by zinc and dilute sulfuric acid (distinction from phosphorous acid). Dehydration of hypophosphoric acid does not yield phosphorus tetroxide; hydration of phosphorus tetroxide does not yield hypophosphoric acid but phosphorous plus phosphoric acids.
Acid sodium salts in baking powders.
ChEBI: Hypodiphosphoric acid is a phosphorus oxoacid.
Hypophosphoric acid is formed by reaction (1) of yellow phosphorous and potassium permanganate in sodium hydroxide medium, (2) of red phosphorus and calcium hypochlorite solution, (3) also one of the products of slow oxidation at ordinary temperatures of phosphorus in moist air.