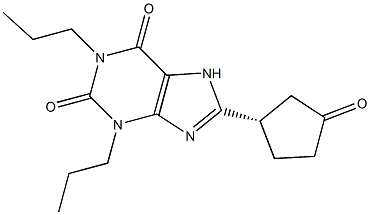
1,3-Dipropyl-8-(3-oxocyclopentyl)xanthyne:
1). 100 g 3-oxocyclopentancarboxylic acid methyl ester (0.7 moles) was
heated with 1000 ml of 2 M hydrochloric acid to reflux for 10 hours. On
cooling the solution was evaporated in vacuum. Residual water was removed
by azeotropic dehydratation. The residue was distilled in high vacuum to give
3-oxocyclopentancarboxylic acid as colorless oil, b.p.: 116°-121°C/0.002 mm;
yield 74.0 g (82 %).
2). 11.6 g carbonyl diimidasole was added to 8.8 g 3-
oxocyclopentancarboxylic acid (0.072 moles) in 240 ml methylene chloride at
20°-25°C and stirred for 2 hours at room temperature. Then 16.0 g 1,6-
diamino-1,3-di-n-propyluracil (0.072) at 20°-25°C was added and was stirred
3 hours at room temperature. The mixture was evaporated in vacuum to
dryness. The residue oil was diluted with 3200 ml water, 35 g of calcium
hydroxide was added and all taken was heated at 80°C for 0.5 hour by
stirring. The mixture was cooled to 5°C, acidified with pH 1-2 with
hydrochloric acid and extracted with CH2Cl2 (3x100 ml). The organic phase
was washed with 1x100 ml water and dried over magnesium sulfate. The
solution was purified with of 350 g silica gel S 160. CH2Cl2 : CH3OH (99:1)
was used as eluent. Refined residue was evaporated and treated with 100 ml
ether. 11.5 g 1,3-dipropyl-8-(3-oxocyclopentyl)xanthyne (apaxifylline)
obtained (50.2 %). MP: 164-168°C.