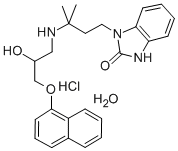
A mixture consisting of 3 g of 1-(3-amino-3,3-dimethyl-n-propyl)
benzimidazolidinone-2, 3.3 g of 1-[naphthyl-(1)-oxyl]propylene-(2,3)-epoxide
and 12 ml of 98% ethanol were refluxed for three hours. Thereafter, the
ethanol was distilled off, the residue was taken up in some methanol, and the
solution was acidified with 1 N hydrochloric acid and then extracted with ethyl
acetate. The ethyl acetate was distilled out of the extract solution, and ether
and some water were added to the residue, whereupon a crystalline substance
separated out. The product was recrystallized from ethanol, yielding 60% of
theory of ()-1-(3-((2-hydroxy-3-(1-naphthyloxy)propyl)amino)-3-
methylbutyl)-2-benzimidazolinone, which had a melting point of 161°C.
In practice it is usually used as hydrochloride.