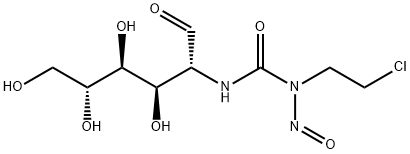
Chlorozotocin is a nitrosourea compound that exists as ivory-coloredcrystals at room temperature. It is soluble in water and is stable in solution at room temperature for up to 3 hours and under refrigeration for 24 hours. The powder form of chlorozotocin is stable under refrigeration for two years. The spontaneous, nonenzymatic degradation of chlorozotocin results in formation of DNA-alkylating and protein-carbamoylating moieties (Chabner et al. 2001).
Chlorozotocin is a cytostatic agent that has been used to treat melanoma and multiple myeloma and cancer of the stomach, large intestine, pancreas, and lung (IARC 1990).
ChEBI: Chlorozotocin is an amino sugar.
Confirmed carcinogen
with experimental carcinogenic and
tumorigenic data. Poison by subcutaneous,
intravenous, and intraperitoneal routes.
Human systemic effects by intravenous
route: anorexia, leukopenia, nausea or
vomiting, thrombocytopenia. Mutation data
reported. When heated to decomposition it
emits very toxic fumes of Cland NOx. See
also NITROSAMINES.
Chlorozotocin is reasonably anticipated to be a human carcinogenbased on sufficient evidence of carcinogenicity from studies in experimental animals and because it is a member of a well-defined, structurally related class of substances listed in the Report on Carcinogensas either known to be a human carcinogen or reasonably anticipated to be a human carcinogen.