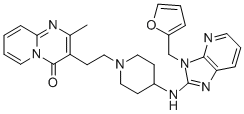
Barmastine
- Product NameBarmastine
- CAS99156-66-8
- MFC27H29N7O2
- MW483.573
- EINECS
- MOL File99156-66-8.mol
Usage And Synthesis
A mixture of N-(3-nitro-2-pyridinyl)-2-furanylmethanamine, of a solution of
thiophene in ethanone 4% and of methanol saturated with ammonia was
hydrogenated at normal pressure and at room temperature with platinum-on-charcoal catalyst 5%. After the calculated amount of hydrogen was taken up,
the catalyst was filtered off and the filtrate was evaporated. The residue was
crystallized from acetonitrile, yielding the N2-(2-furanylmethyl)-2,3-
pyridinediamine.
A mixture of 54 parts of ethyl 4-isothiocyanato-1-piperidinecarboxylate, 48 parts of N2-(2-furanylmethyl)-2,3-pyridinediamine and 450 parts of tetrahydrofuran was stirred and refluxed overnight. The reaction mixture was evaporated and the residue was crystallized from a mixture of 2-propanone and 2,2'-oxybispropane. The product was filtered off and dried, yielding 76 parts (75%) of ethyl 4-[[[2-[(2-furanylmethyl)amino]-3- pyridinyl]aminothioxomethyl]amino]-1-piperidinecarboxylate; melting point 132.7°C.
A mixture of 74 parts of ethyl 4-[[[2-[(2-furanylmethyl)amino]-3- pyridinyl]aminothioxomethyl]amino]-1-piperidinecarboxylate, 96 parts of mercury (II) oxide, 0.1 parts of sulfur and 800 parts of ethanol was stirred and refluxed for 3 h. The reaction mixture was filtered over Hyflo and the filtrate was evaporated to give 52.5 parts (79%) of ethyl 4-[[3-(2- furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-yl]amino]-1-piperidinecarboxylate; melting point 149.2°C (crystallized from acetonitrile).
A mixture of ethyl 4-[[3-(2-furanylmethyl)-3H-imidazo[4,5-b]pyridin-2- yl]amino]-1-piperidinecarboxylate and of a hydrobromic acid solution 48% in water was stirred and heated for 3 h at about 80°C. The reaction mixture was evaporated and the residue was dried, yielding the 3-(2-furanylmethyl)-N-(4- piperidinyl)-3H-imidazo[4,5-b]pyridin-2-amine dihydrobromide (crystallized from methanol).
A mixture of 3-(2-hydroxyethyl)-2-methyl-pyrido[2,1-b]pyrimidin-4-one, of acetic acid and of a hydrobromic acid solution 67% in acetic acid was stirred and heated to reflux. Stirring was continued overnight at reflux temperature. The reaction mixture was evaporated and the solid residue was triturated in 2- propanone. The product was filtered off and dried, yielding 3-(2-bromoethyl)- 2-methyl-pyrido[1,2-a]pyrimidin-4-one monohydrobromide.
A mixture of 3-(2-bromoethyl)-2-methyl-pyrido[1,2-a]pyrimidin-4-one monohydrobromide, 3-furan-2-yl-methyl-(3H-imidazo[4,5-b]pyridine-2yl)-4- piperidinyl)-amine dihydrobromide, of sodium carbonate and of N,Ndimethylformamide was stirred and heated overnight at about 70°C. The reaction mixture was poured onto water. The product was extracted with trichloromethane. The extract was dried, filtered and evaporated. The residue was purified by column chromatography over silica gel using a mixture of trichloromethane and methanol (94:6 by volume), saturated with ammonia, as eluent. The pure fractions were collected and the eluent was evaporated. The residue was crystallized from acetonitrile, yielding 3-(2-(4-((3-(2- furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-yl)amino)-1-piperidinyl)ethyl)-2- methyl-4H-pyrido[1,2-a]pyrimidin-4-one; melting point 202°C.
A mixture of 54 parts of ethyl 4-isothiocyanato-1-piperidinecarboxylate, 48 parts of N2-(2-furanylmethyl)-2,3-pyridinediamine and 450 parts of tetrahydrofuran was stirred and refluxed overnight. The reaction mixture was evaporated and the residue was crystallized from a mixture of 2-propanone and 2,2'-oxybispropane. The product was filtered off and dried, yielding 76 parts (75%) of ethyl 4-[[[2-[(2-furanylmethyl)amino]-3- pyridinyl]aminothioxomethyl]amino]-1-piperidinecarboxylate; melting point 132.7°C.
A mixture of 74 parts of ethyl 4-[[[2-[(2-furanylmethyl)amino]-3- pyridinyl]aminothioxomethyl]amino]-1-piperidinecarboxylate, 96 parts of mercury (II) oxide, 0.1 parts of sulfur and 800 parts of ethanol was stirred and refluxed for 3 h. The reaction mixture was filtered over Hyflo and the filtrate was evaporated to give 52.5 parts (79%) of ethyl 4-[[3-(2- furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-yl]amino]-1-piperidinecarboxylate; melting point 149.2°C (crystallized from acetonitrile).
A mixture of ethyl 4-[[3-(2-furanylmethyl)-3H-imidazo[4,5-b]pyridin-2- yl]amino]-1-piperidinecarboxylate and of a hydrobromic acid solution 48% in water was stirred and heated for 3 h at about 80°C. The reaction mixture was evaporated and the residue was dried, yielding the 3-(2-furanylmethyl)-N-(4- piperidinyl)-3H-imidazo[4,5-b]pyridin-2-amine dihydrobromide (crystallized from methanol).
A mixture of 3-(2-hydroxyethyl)-2-methyl-pyrido[2,1-b]pyrimidin-4-one, of acetic acid and of a hydrobromic acid solution 67% in acetic acid was stirred and heated to reflux. Stirring was continued overnight at reflux temperature. The reaction mixture was evaporated and the solid residue was triturated in 2- propanone. The product was filtered off and dried, yielding 3-(2-bromoethyl)- 2-methyl-pyrido[1,2-a]pyrimidin-4-one monohydrobromide.
A mixture of 3-(2-bromoethyl)-2-methyl-pyrido[1,2-a]pyrimidin-4-one monohydrobromide, 3-furan-2-yl-methyl-(3H-imidazo[4,5-b]pyridine-2yl)-4- piperidinyl)-amine dihydrobromide, of sodium carbonate and of N,Ndimethylformamide was stirred and heated overnight at about 70°C. The reaction mixture was poured onto water. The product was extracted with trichloromethane. The extract was dried, filtered and evaporated. The residue was purified by column chromatography over silica gel using a mixture of trichloromethane and methanol (94:6 by volume), saturated with ammonia, as eluent. The pure fractions were collected and the eluent was evaporated. The residue was crystallized from acetonitrile, yielding 3-(2-(4-((3-(2- furanylmethyl)-3H-imidazo[4,5-b]pyridin-2-yl)amino)-1-piperidinyl)ethyl)-2- methyl-4H-pyrido[1,2-a]pyrimidin-4-one; melting point 202°C.
Preparation Products And Raw materials
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine