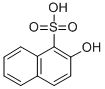
2-Hydroxynaphthalene-1-sulfonic acid [567-47-5]. oxy Tobias acid, C10H8O4S, Mr 224.2, and its salts are very soluble in water; neutral solutions give a strong blue color with iron(III) chloride. The sulfonic acid group is sufficiently labile for desulfonation to take place slowly in aqueous solution and rapidly with diazo compounds to form 1-azo-2-hydroxynaphthalene derivatives (i.e., the same product as from 2-naphthol), and with bromine to form 1-bromo2-hydroxynaphthalene. Low-temperature sulfonation gives 2-hydroxynaphthalene-1,6-disulfonic acid, whereas higher temperatures result in 1- desulfonation and formation of 2-hydroxynaphthalene-6- and 8-sulfonic acids. Nitration affords 1,6-dinitro-2-hydroxynaphthalene.
Virtually all the 2-hydroxynaphthalene-1-sulfonic acid produced is converted to 2-aminonaphthalene-1-sulfonic acid (Tobias acid) by amination. The calcium salt Asaprol is soluble in aqueous ethanol and has been used in place of gypsum in surgical plaster.
2-Naphthol is dissolved in 1,2- dichloroethane and chlorosulfonic acid is added at 0 ℃;the reactionis allowed to goto completion at low temperature. During work-up and isolation, desulfonation is avoided by careful extraction of the product into aqueous alkali and salting out of the sodium salt. Alternative solvents, such as dichloromethane, tetrachloroethane, or 1,2-dichlorobenzene, and other sulfonating agents, (e.g., liquid sulfur trioxide) can be used.