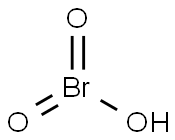
colorless or slightly yellowish liquid; stable only in very dilute aqueous solutions; oxidizing agent; used in dyes [HAW93]
Unstable compound; stable only in dilute aqueous solutions; solution turns yellow on standing; decomposes when heated to 100°C.
Bromic acid is prepared by adding sulfuric acid to barium bromate.
Ba(BrO3)2 + H2SO4 → 2HBrO3 + BaSO4
The product is distilled and absorbed in water. A 50% solution may be obtained by slow evaporation of the dilute solution in vacuum at -12°C.
ChEBI: Bromic acid is a bromine oxoacid. It is a conjugate acid of a bromate.
Contact with skin and eyes can cause severe irritation.