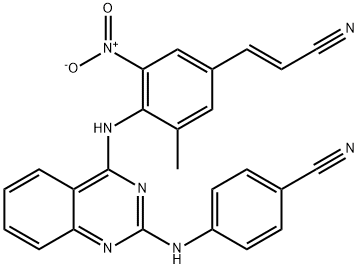
Reverse transcriptase-IN-1 (Compound 12z), a diarylbenzopyrimidine (DABP) analogue, is a potent, orally active HIV-1 nonnucleoside reverse transcriptase inhibitor. Reverse transcriptase-IN-1 has antiviral activity with EC50 values of 3.4 nM, 4.3 nM and 3.6 nM for HIV-1 IIIB, E138K and K103N mutants, respectively. Reverse transcriptase-IN-1 also has an IC50of 13.7 nM against HIV-1 reverse transcriptase enzyme[1].
The oral bioavailability of Reverse transcriptase-IN-1 (Compound 12z) is significantly improved to 16.5% at a dose of 5 mg/kg in rats. The intrinsic rat microsome clearance of Reverse transcriptase-IN-1 is 33.2 μL/min/mg proteins. The PK study and safety assessment of Reverse transcriptase-IN-1 shows that it is absorbed with mean residence times (MRTs) of 11.8 hours (5 mg/kg, p.o.) and 11.4 hours (1 mg/kg, i.v.) at these two doses. The Cmax of Reverse transcriptase-IN-1 is 39.9 ng/mL at a dose of 5 mg/kg. A single-dose toxicity test of Reverse transcriptase-IN-1 in rats shows no mortality, and there is no abnormal body weight decrease in the animals in the week following an intragastrical dose at 293 mg/kg body weigh. The above results indicate that Reverse transcriptase-IN-1 could be an orally bioavailable candidate for human HIV-1 infection research[1].
[1]. Han S, et al. Molecular Hybridization-Inspired Optimization of Diarylbenzopyrimidines as HIV-1 Nonnucleoside Reverse Transcriptase Inhibitors with Improved Activity against K103N and E138K Mutants and Pharmacokinetic Profiles. ACS Infect Dis. 2019 Oct 24.