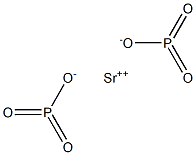
Strontium hypophosphate would have the molecular
formula of Sr2P2O4, if it were to be produced. It can be
formed by an aqueous solution of hypophosphorus
acid and a soluble strontium salt:
2MgCl2 (aq) +H4P2O4 (aq) ? Mg2P2O4 (solid)
+ 2HCl (aq)
The strontium salt solution is added until no
precipitate results. Alternatively, sodium hypophosphate
can be used to produce a basic solution instead
of acidic.
However, there is categorically norecordofpreparation
of this salt, unlike that of barium. It would be expected
to mimic the properties of the homologous barium
salts but no such descriptions can be found in any of
the textbooks or scientific literature concerning the properties
of this salt. Whether it forms hydrates or an acid
salt remains unknown. It is expected to be rather insoluble
in water but soluble in acids. It does not have a CAS
number.