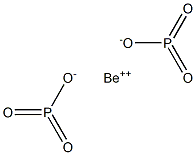
Beryllium hypophosphate should have the molecular
formula of Be2P2O6 if it were to be formed by a solidstate
reaction between hypophosphoric acid and BeO:
BeO +H4P2O6 + heat ? Be2P2O6 + 2H2O
However, there is no record that the preparation of
this salt has ever been attempted. It could also be formed
via an aqueous solution of hypophosphorus acid and
a soluble beryllium salt:
2(BeOH)Cl (aq) +H4P2O4 (aq) ? (BeOH)2P2O4 (solid)
+ 2HCl (aq)
Alternatively, sodium hypophosphate could be used
to produce a basic solution instead of an acidic pH. A
product from a basic solution, prepared in 1916, was
said to have the formula, Be2P2O6·1.5H2O but this has
never been confirmed. This compound was stated to
lose its waters of hydration between 230 and 240 °C.
The formation of an “acid” salt-like BeH2P2O6 or
(BeOH)H2P2O6 remains speculative.