20 g of 1-(1-chlorobutyl)-4-methoxybenzene is dissolved in 50 ml of ether
which had been dried over sodium. Separately, 2.8 g of magnesium turnings
are covered with 20 ml of ether. 0.2 g of iodine, as a catalyst, is added and
the solution of 1-(1-chlorobutyl)-4-methoxybenzene is added at such a rate as
to keep the ether refluxing gently. If there action dopes not start immediately
after the addition of a few drops of the halide, the solution may be heated
cautiously in order to start the reaction. The mixture is then refluxed for
another 0.5 hour and then allowed to cool down. During the whole reaction a
current of hydrogen or nitrogen is passed through the apparatus. The
resulting grignard reagent is filtered, cooled to -10°C and added slowly to a
solution of 18 g of 1-(methoxyphenyl)-propan-1-one in 20 ml benzene to
which 0.2 g of MnCl2. The grignard reagent is added so slowly that the
temperature is kept below 0°C. After 2 hours, the temperature is raised to
room temperature and the solvent distilled off in vacuo. The residue is heated
to 170°C at a pressure of 0.4 mm Hg. MgCl(OH) is split off and the product
distils over. This compound is dealkylated by heating it for 20 hours with a
solution of KOH in glycerin at 190°C in an atmosphere of nitrogen to obtain
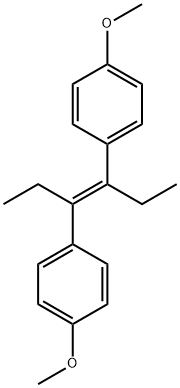
α,α-diethyl-4,4'-dimethoxystilbene.