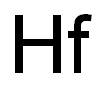Hafnium hydride is formed as refractory, tetrahedral crystals.
Hafnium hydride is usually produced as an intermediate in
the process of making hafnium powder from mass hafnium
metal. Hafnium reacts reversibly with hydrogen to form
hydride, and the proportion of hydrogen depends upon the
temperature and pressure of hydrogen used. As hydrogen is
absorbed, the hafnium transforms from the hexagonal metal
to the face-centered cubic hydride and then to the facecentered
tetragonal hydride. Hafnium hydride is brittle
and is easily crushed to very fine particles sizes.