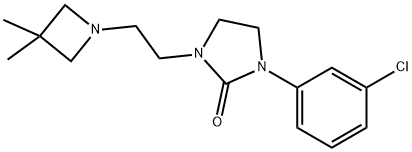
A solution of 5.0 g of 1-(m-chlorophenyl)-2-imidazolidinone in 30 ml of
dimethylformamide is added at room temperature to a mixture of 1.5 g of
50% NaH (mineral oil emulsion) in 30 ml of dimethylformamide. The reaction
mixture is stirred for 1 h at room temperature and then 4.5 g of 3,3-dimethyl-
1-(2-chloroethyl)azetidine are added. The mixture is stirred again at room
temperature for 2 h and then it is heated for 5 h at 80°-85°C. The salts are
filtered off and the solvent is removed under vacuum. The residue is taken up
with 8 ml of 18% HCl and 16 ml of water and extracted with diethyl ether.
The water solution is alkalinized by addition of 15% sodium carbonate and
then extracted with diethyl ether. By evaporation of the organic layer a
residue is obtained that is triturated in light petroleum. Yield of 1-(m-
chlorophenyl)-3-[2-(3,3-dimethylazetidin-1-y1)ethyl]-2-imidazolidinone 7.25
g, melting point 84°-85°C (after recrystallization from hexane).