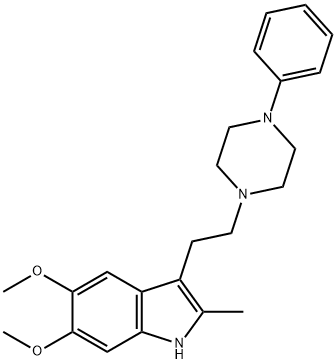
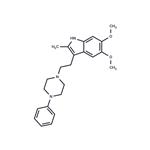
A cold, stirred solution of 1-phenyl-piperazine in tetrahydrofuran was treated all at once with [3-(2-methyl-5,6-dimethoxy)indolyl]glyoxalyl chloride. There was an immediate voluminous precipitate of a white crystalline solid which was removed by filtration. The filtrate was taken to dryness and the residual light brown gum was stirred and shaken with water, ethyl acetate and acetic acid. The mixture was warmed on a steam bath and the resulting solid was collected after cooling in an ice bath thus affording 1-[(3-(2-methyl-5,6dimethoxy)indolyl)glyoxalyl]-4-phenylpiperazine as a near white solid, melting point 163°-174°C.
A solution of 1-[(3-(2-methyl-5,6-dimethoxy)indolyl)glyoxalyl]-4-phenyl piperazine in tetrahydrofuran was added over a 10 min period to a stirred suspension of lithium aluminum hydride in tetrahydrofuran. The mixture was refluxed and stirred for 6.5 h and the excess lithium aluminum hydride then destroyed by the dropwise addition of 10% sodium hydroxide solution. The mixture was filtered, the insoluble material was washed with boiling chloroform, and the filtrate dried over anhydrous sodium sulfate and concentrated to dryness. The residual light orange oil was crystallized from a benzene-hexane mixture giving 1-[(3-(2-methyl-5,6-dimethoxy)indolyl)ethyl]4-phenyl piperazine.