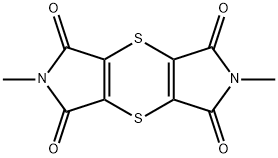
ChEBI: Dipymetitrone is an organonitrogen heterocyclic compound that is 1H,5H-[1,4]dithiino[2,3-c:5,6-c']dipyrrole-1,3,5,7(2H,6H)-tetrone substituted at positions 2 and 6 by methyl groups. It has a role as an antifungal agrochemical. It is an organic heterotricyclic compound, a dicarboximide fungicide, an organosulfur pesticide, an organosulfur heterocyclic compound and an organonitrogen heterocyclic compound. It is functionally related to a maleimide.
Dipyridamole appears to act in vivo by synergistically modifying several biochemical pathways, including:
(1) inhibition of platelet cAMP-phosphodiesterase;
(2) potentiation of adenosine inhibition of platelet function by blocking reuptake by vascular and blood cells, and subsequent degradation of adenosine; and possibly;
(3) potentiation of PGI2 antiaggregatory activity and enhancement of PGI2 biosynthesis.
These independent processes inhibit platelet function by increasing platelet cAMP through both a reduction in enzymatic cAMP-degradation, and stimulation of cAMP formation via activation of adenylcyclase by adenosine and possibly PGI2.
Dipymetitrone is a Dicarboximide fungicide that can be used as a pesticide chemical, any substance used to control, prevent or destroy animal, microbial or plant pests. It is also used as any substance for aquaculture, horticulture, forestry, etc.
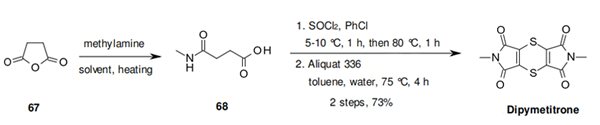
Dipymetitrone is synthesised as follows:
The synthesis of dipymetitrone could start from the reaction of
methylamine and succinic anhydride (67) to afford 68. Treatment of 68 with thionyl chloride and
isomerization of the intermediate diisomaleimide-dithiine with water yields dipymetitrone.