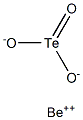
Berylliumtellurite has themolecular formula of BeTeO3
and the molecular weight of 184.61 grams per mole. It
can be prepared by means of the solid-state reaction:
BeO+ TeO2+ heat ? BeTeO3
An inert atmosphere is required. This salt is a metatellurite
but its formation and physical properties have
not been reported in the literature. If a solution method
is used, the result is likely to be quite different in that
a hydroxy-tellurite is formed:
BeCl2+ Na2TeO3 ? [Be(OH)]2TeO3
Additionally, this compound can be prepared by
reacting tellurium dichloride in a solution of beryllium
chloride:
BeCl2
+ 2TeCl2
+ 4H2O ? Te+ [Be(OH)]2TeO3
+ 6HCl
Nonetheless, there is absolutely no description of
these compounds in the literature and whether a hydrate
appears remains unknown.