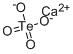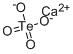Calcium tellurate has the molecular formula of
CaTeO4 and the molecular weight of 231.68 grams per
mole. Its CAS number is 712354-41-1 and its solubility
in water is 0.727 g/100 ml at 20°C. It can be prepared
by the solid-state reaction of TeO3 and CaO:
CaO + TeO3 + heat ? CaTeO4
TeO3 melts at 430 °C and a temperature of 600° C serves
to drive the reaction to completion. This is the tetragonal
meta-tellurate composition. It can also be formed in the
same manner by solid-state reaction of telluric acid:
CaO(solid)+H2TeO4(solid) ? CaTeO4(solid)+H2O(gas)
This is also tetragonal in structure, with the meta-tellurate
configuration. Of the two possible configurations,
the meta-tellurate is said to be the most stable.
Telluric acid is soluble in water and produces
Te(OH)4O2
anions in solutions.
Calcium tellurate has been used to isolate telluric acid
by heating TeO2 with and excess of CaO at 975°C in air
at a ration of 1:5 equivalents. The dioxide was completely
oxidizedandtheproductwasCaTeO4.Ratiosless thanthis
affected the overall yield. By adding nitric acid, soluble
calcium nitrate and insoluble telluric acid were formed:
5CaO(solid) + TeO2(solid) + heat?CaTeO4(solid)
CaTeO4(solid) +HNO3(aq)?Ca(NO3)2(aq)
+H2TeO4(solid)
If calcium tellurite(IV) is heated, in N2 atmosphere,
with stoichiometric amounts of CaO, the reaction
product, proceeding at 380–620°C, contained orthotellurate
and telluride anions formed in disproportionation:
3CaTeO3 + CaO + heat?CaTeO4 + 2CaTe
The scientific literature concerning calcium tellurate
is exceedingly limited and no data on its physical properties
have been published. It has found little usage in
industry but is available commercially in small quantities
for use in research.