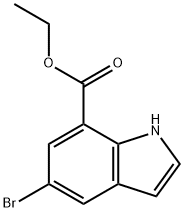
Intermediate 4: Synthesis of ethyl 5-bromo-1H-indole-7-carboxylate. Ethyl 2,3-dihydro-5-bromo-1H-indole-7-carboxylate (129.1 g, 0.48 mmol) was dissolved in chloroform (1500 mL) and DDQ (119.3 g, 525.7 mmol) was added in batches. The reaction mixture was stirred at room temperature for 2 hours. After completion of the reaction, the mixture was filtered and the solids were washed with chloroform (3 x 500 mL). The combined filtrates were washed sequentially with 5% sodium hydroxide solution (3 x 500 mL), water (500 mL), and saturated brine (500 mL) and then dried with anhydrous sodium sulfate. The filtrate was concentrated under reduced pressure and the residue was recrystallized from ethanol to give ethyl 5-bromo-1H-indole-7-carboxylate (88 g, 69% yield). The product was analyzed by LC/MS: m/z 267.6 (M+H), retention time 1.14 min.