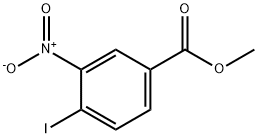
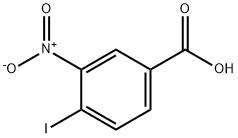
Example 1: General procedure for the preparation of methyl 4-iodo-3-nitrobenzoate using methanol/sulfuric acid
1. dissolve 4-iodo-3-nitrobenzoic acid (3 g, 10 mmol) in methanol (30 ml) and cool the solution to 0°C.
2. sulfuric acid (3.4 g, 34.6 mmol) was added slowly to the above cooled solution.
3. the reaction mixture was gradually warmed to room temperature and subsequently heated to reflux (about 70 °C) and maintained for 8 hours.
4. Upon completion of the reaction, the reaction mixture was cooled and neutralized to pH neutral with solid NaHCO3 and filtered to remove the resulting salt.
5. The filtrate was concentrated under pressure to give a crude product.
6. Water (30 ml) was added to the crude product and extracted with methyl tertiary butyl ether (MTBE, 30 ml x 2).
7. The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate and filtered.
8. The solvent was evaporated under pressure to give methyl 4-iodo-3-nitrobenzoate as a yellow solid (2.67 g, 85% yield, 98% HPLC purity).
[1] Dalton Transactions, 2015, vol. 44, # 5, p. 2047 - 2051
[2] Chemical Communications, 2017, vol. 53, # 55, p. 7808 - 7811
[3] Patent: US2013/172618, 2013, A1. Location in patent: Paragraph 0025
[4] Patent: WO2017/48954, 2017, A1. Location in patent: Paragraph 00095
[5] Patent: WO2005/40157, 2005, A2. Location in patent: Page/Page column 68