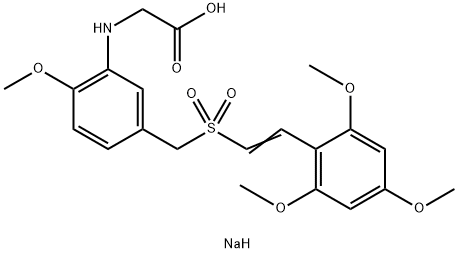
Rigosertib (ON-01910) is a non-ATP-competitive inhibitor of Polo-like kinase 1 (PLK1).
ChEBI: Rigosertib sodium is the sodium salt of rigosertib. It is an anti-cancer agent which has been granted Orphan Drug Designation by the FDA for use in patients with myelodysplastic syndromes (MDS). It has a role as a microtubule-destabilising agent, an antineoplastic agent, an EC 2.7.11.21 (polo kinase) inhibitor and an apoptosis inducer. It contains a rigosertib(1-).
rigosertib (on-01910,estybon) is a potent, specific plk1 inhibitor with ic50 value of 9nm. rigosertib strongly inhibited the proliferation of cancer cell lines, with observed ic50 values in the nanomolar range for both hela (115 nm) and c33a (45 nm) cells. in contrast, rigosertib had a minimal effect on normal cell lines, bj and ect1/e6e7 (ic50 > 0.1 mm) [1]hela and c33a cells demonstrated a complete (>95%) g2/m arrest at concentrations of rigosertib >0.5 μm, whereas atrigosertib has been reported to be a more potent radiosensitizer than cisplatin in vivo [1].
[1] agoni l1, basu i2, gupta s3, alfieri a2, gambino a4, goldberg gl5, reddy ep6, guha c7.rigosertib is a more effective radiosensitizer than cisplatin in concurrent chemoradiation treatment of cervical carcinoma, in vitro and in vivo. int j radiat oncol biol phys. 2014 apr 1;88(5):1180-7.