Usage And Synthesis
(a) Preparation of 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine: 10 g of 2-
ethyl-3-hydroxy-3,3-diphenyl-propionitrile are dissolved in 200 ml of
methanol. 10 ml of acetic acid are added to the mixture, and the mixture is
hydrogenated in the presence of platinum as catalyst. After the hydrogen
uptake or consumption has ceased, the reaction is interrupted, the catalyst is
filtered off and the filtrate is evaporated in vacuo to dryness. The residue is
dissolved in water and, after the addition of 1 ml of hydrochloric acid, the
solution extracted with ether. The acidified ether-phase is discarded. The
aqueous phase is made alkaline with ammonia, whereby the base crystallizesout. The crystals are recovered and recrystallized from methanol. The melting
point of the 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine thereby obtained is
132°C.
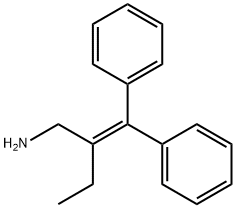
(b) Preparation of 2-ethyl-3,3-diphenyl-1-amino-propene-(2)-hydrochloride: 5 g of 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine are dissolved in 50 ml of acetic acid. Gaseous hydrogen chloride is passed through the solution for 10 minutes, and thereafter the solution is boiled for one hour under reflux. The solution is then distilled to dryness. The residue is dissolved in water and the acidified solution extracted with ether. The aqueous phase is separated, made alkaline with ammonia and extracted with ether. The ether phase is dried over sodium sulfate, the ether distilled off and the residue is dissolved in methanolic hydrogen chloride. On the addition of absolute ether, the hydrochloride of 2-ethyl-3,3-diphenyl-1-amino-propene-(2) is crystallized out. The crystalline substance thereby obtained has a melting point of 232°C.
(b) Preparation of 2-ethyl-3,3-diphenyl-1-amino-propene-(2)-hydrochloride: 5 g of 2-ethyl-3-hydroxy-3,3-diphenyl-propylamine are dissolved in 50 ml of acetic acid. Gaseous hydrogen chloride is passed through the solution for 10 minutes, and thereafter the solution is boiled for one hour under reflux. The solution is then distilled to dryness. The residue is dissolved in water and the acidified solution extracted with ether. The aqueous phase is separated, made alkaline with ammonia and extracted with ether. The ether phase is dried over sodium sulfate, the ether distilled off and the residue is dissolved in methanolic hydrogen chloride. On the addition of absolute ether, the hydrochloride of 2-ethyl-3,3-diphenyl-1-amino-propene-(2) is crystallized out. The crystalline substance thereby obtained has a melting point of 232°C.
Preparation Products And Raw materials
Raw materials
Related Product Information
- florfenicol(Water soluble)
- Florfenicol
- Piroheptine
- prifinium bromide
- 3-ACETYL-6-BROMO-4-PHENYLQUINOLIN-2(1H)-ONE
- HSR 405
- LABOTEST-BB LT00012623
- Pridefine
- LK-190
- 6-CHLORO-3-[(1E)-ETHANEHYDRAZONOYL]-4-PHENYLQUINOLIN-2(1H)-ONE
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine