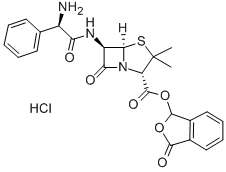
Talampicillin was synthesized by Yamanouchi Pharmaceutical Co. in 1971 by esterifying the carboxylic acid group of ampicillin in order to improve the oral absorption. When administered orally, it is hydrolyzed to ampicillin by intestinal esterase. Its bioavailability is two or more times as high as that of ampicillin, and it is used to treat the same infections as those for which ampicillin is used orally but in doses only half or one-third as large.
A fine suspension of 25.18 grams (0.05 mol) of potassium salt of enamine
protected ampicillin and 10.65 grams (0.05 mol) 3-bromophthalide were
reacted in a 1:2 mixture of acetone/ethyl acetate (1,500 ml) for 24 hours.
After filtration the organic layer was washed twice with 250 ml portions of 1 N
sodium bicarbonate and brine, dried over anhydrous magnesium sulfate and
concentrated in vacuo. Addition of ether crystallized the phthalide enamine
protected α-aminophenylacetamido penicillanate in 85% yield.