Into a 3-necked spherical flask of 1 L provided with a dropping funnel, a
condenser surmounted by a calcium chloride tube and a mechanical stirrer, is
introduced a suspension of 17.6 g (0.2 mol) of ethyl urea in 150 ml of
anhydrous benzene. There is added through the dropping funnel in 20 minutes
a solution of 18.9 g (0.1 mol) α-chlorophenyl-acetyl chloride in 300 ml of
benzene. The mixture is left at ambient temperature for 15 minutes and is
then heated under reflux on the water bath with stirring for 5 hours. The
benzene solution is decanted at elevated temperature in order to separate it
from an oil deposited on the bottom of the flask, the benzene is driven off on
the water bath, the last traces removed in vacuum, the crystalline residue is
triturated in a mortar in about 200 ml of water, and the crystalline solid is
separated off and is with water and dried in vacuum over phosphorus
pentoxide. There are thus recovered 19.6 g (yield 82%) of 1-(2-chloro-2-
phenyl-acetyl)-3-ethyl-urea, which when recrystallized from 80 ml of benzene,
takes the form of white crystals soluble in benzene but insoluble in water. The
product has melting point 146°C. To a suspension of 39 g (0.163 mol) of 1-(2-chloro-2-phenyl-acetyl)-3-ethylurea
in 250 ml of anhydrous ethyl alcohol is added a sodium ethoxide solution
containing 3.75 g (0.163 mol) of sodium dissolved in 250 ml of ethyl alcohol.
The mixture is heated under reflux for 2 hours and left overnight at ambient
temperature. The precipitated sodium chloride is separated off and copiously
washed with alcohol. The alcohol is driven off from the filtrate on the water
bath, the oily residue is triturated in 20 ml of iced water, and the solid formed
is refrigerated for several hours, separated, washed with water and dried in
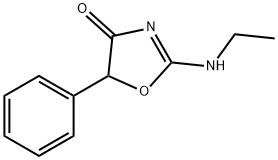
vacuum over phosphorus pentoxide. The 5-phenyl-2-ethylamino-4-oxazolinone
(fenozolone) obtained is recrystallized from anhydrous benzene. It then forms
a white crystalline compound soluble in benzene and insoluble in water, MP:
148°C.