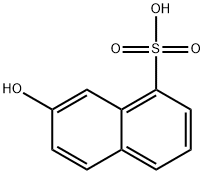
Colorless crystals. Easily soluble in water and alcohol. 2-Hydroxynaphthalene-8-sulfonic acid [132-57-0]. (7-hydroxynaphthalene-1-sulfonic acid), crocein acid, Bayer’s acid, C10H8O4S, Mr 224.23, is more soluble in water than 2-hydroxynaphthalene-6-sulfonic acid and cannot readily be isolated. Its salts are also more soluble than their 2,6-analogues and are difficult to isolate. On evaporation of its solutions, the free acid (2-Hydroxynaphthalene-8-sulfonic acid) decomposes to 2-naphthol. Coupling with diazo compounds takes place in the 1-position, but reaction is hindered by the peri-sulfonic acid group so that concentrated conditions and sometimes catalysts are required. Above 60 ℃, sulfuric acid converts 2-Hydroxynaphthalene-8-sulfonic acid into 2-hydroxynaphthalene-6-sulfonic acid; sulfonation then proceeds further to give the 6,8-disulfonic acid. Amination (Bucherer reaction) gives 2-aminonaphthalene8-sulfonic acid.