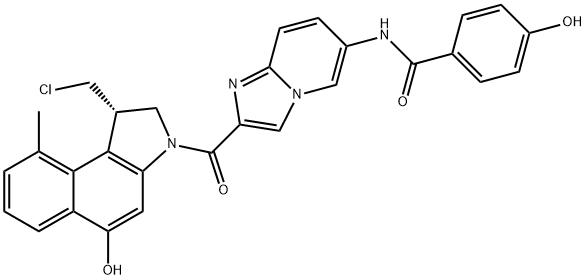
Seco-DUBA is a inactive prodrug form of duocarmycin (DUBA) containing two hydroxyl groups, which can each be used for coupling to an antibody via a linker. Seco-DUBA can be used in the synthesis of antibody-drug conjugates (ADCs).
Duocarmycins (DUMs; A, B1, B2, C1, C2, D1, D2 and SA) are members of an extremely potent group of antitumor antibiotics isolated from Streptomyces sp. The pharmacophore consists of a cyclopropa[c]pyrrolo[3,2-e]indole (CPI) moiety. DUMs B1, B2, C1, C2, D1 and D2 were a seco-type of DUM A and chemically more stable than DUM A[1].
Seco-DUBA (SK-BR-3 cells; 0.0001 pM~0.01 nM; 144 hours) dose-dependent reduces cell viability and shows equally potent and efficacious as DUBA[1].
Seco-DUBA causes SK-BR-3 (IC50=0.09), SK-OV-3 (IC50=0.43), and SW620 (IC50=0.09) cells to exhibit highly sensitive.
Seco-DUBA (89 μg/kg; i.v.) is likely converted to DUBA almost instantaneously[2].
Humanized IgG1, HER-2
Humanized IgG1, 5 T4
Humanized IgG1, CD22
Mouse IgG1; CD56
[1] Choi T, et al. Structural Influence of Indole C5-N-Substitutents on the Cytotoxicity
of seco-Duocarmycin Analogs. Archives of Pharmacal Research, 2011; 34: 357–367.
[2] Elgersma R, et al. Design, Synthesis, and Evaluation of Linker-Duocarmycin Payloads: Toward Selection of HER2-Targeting Antibody–Drug Conjugate SYD985. Molecular Pharmaceutics, 2015; 12: 1813–1835.