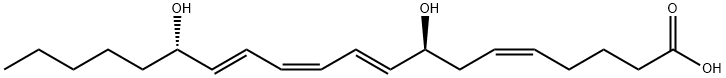
8(S),15(S)-DiHETE is formed when 15(S)-HETE is subjected to further oxidation by 15-LO. It causes eosinophil chemotaxis with an ED50 value of 1.5 μM but is not chemotactic for neutrophils. 8(S),15(S)-DiHETE antagonizes the hyperalgesic activity of 8(R),15(S)-DiHETE and LTB4 in the rat hind paw pain model.
8(S),15(S)-DiHETE (Z, E, Z, E) is a novel dioxygenation product of arachidonic acid.
ChEBI: 8(S),15(S)-DiHETE is ab 8,15-DiHETE that is derived from (5Z,9E,11Z,13E)-icosatetraenoic acid and has (8S,15S)-stereochemistry. It has a role as a human xenobiotic metabolite and a mouse metabolite. It is functionally related to an arachidonic acid. It is a conjugate acid of an 8(S),15(S)-DiHETE(1-).