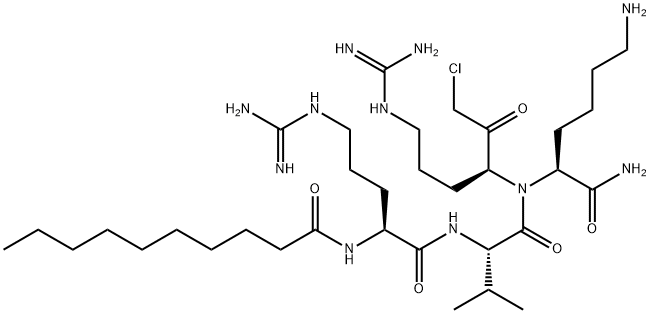
N2-(1-Oxodecyl)-L-arginyl-L-valyl-N-[(1S)-4-[(aminoiminomethyl)amino]-1-(chloroacetyl)butyl]-L-lysinamide is a Furin inhibitor that may be used in the inhibition of the Chikungunya virus (CHIKV), a mosquito-transmitted Alphavirus that causes in humans an acute infection characterized by polyarthralgia, fever, myalgia, and headache
ChEBI: Decanoyl-L-Arg-L-Val-L-Lys-L-Arg-chloromethylketone is a tetrapeptide consisting of N-decanoyl-L-arginine, L-valine, L-lysine, and L-arginine joined in sequence by peptide linkages and in which the carboxy group at the C-terminus is substituted by a chloroacetyl group. It is a furin inhibitor that has antiviral activity against Chikungunya virus, Zika virus and Japanese encephalitis virus. It has a role as an antiviral agent, a peptidomimetic and an EC 3.4.21.75 (furin) inhibitor. It is a tetrapeptide and an organochlorine compound.
decanoyl-rvkr-cmk is a proprotein convertase inhibitor [1].proprotein convertases (pcs) are involved in generating bioactive peptides, as well as activating several enzymes and growth factors in many important physiological events. pcs play important roles in several pathologies including viral infections and cancers. thus, pcs are promising targets for therapeutic applications [1].in pc12 cells, the pc enzyme inhibitor decanoyl-rvkr-cmk at ~ 100 μm, resulted in partial block of regulated vgf release at 48 h and a complete block at 96 h, without significant change in basal vgf release. also, decreased vgf processing was observed in cell extracts and media from decanoyl-rvkr-cmk-treated pc12 cultures [2].in k5-pace4 transgenic mice topically treated with the hyperplasiogenic phorbol ester 12-o-tetradecanoylphorbol-13-acetate (tpa), a 2-day topical treatment of decanoyl-rvkr-cmk at 300 μm, inhibited tpa-induced epidermal proliferation. when decanoyl-rvkr-cmk treatment (100 μm, q.d.) was extended for 3 weeks, decreasing numbers of ki-67-labeled cells were shown in both k5-pace4 mice epidermal basal epidermal keratinocytes and wild-type mouse epidermis [3].[1]. fugère m, day r. cutting back on pro-protein convertases: the latest approaches to pharmacological inhibition. trends in pharmacological sciences, 2005, 26(6): 294-301.[2]. garcia a l, han s k, janssen w g, et al. a prohormone convertase cleavage site within a predicted alpha-helix mediates sorting of the neuronal and endocrine polypeptide vgf into the regulated secretory pathway. journal of biological chemistry, 2005, 280(50): 41595-41608.[3]. bassi d e, zhang j, cenna j, et al. proprotein convertase inhibition results in decreased skin cell proliferation, tumorigenesis, and metastasis. neoplasia, 2010, 12(7): 516-526.