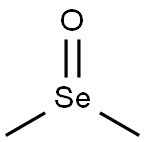
Dimethyl selenoxide can be used as a mild oxidant; an electrophilic reagent for olefin oxidative selenization; and an aromatic substitution electrophilic reagent.
Dimethyl selenoxide is readily obtained by oxidation of dimethyl selenide with 30% aqueous Hydrogen Peroxide at -10 °C, or with Ozone in chloroform at -50 °C; also prepared by treating dimethylselenium dibromide with Silver(II) Oxide in methanol.
Hygroscopic; decomposes in ether, acetonitrile, THF, and CS2; since the reactions of dimethyl selenoxide often produce volatile organoselenium derivatives having a highly unpleasant odor, use in a well-ventilated fume hood.