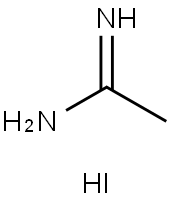
Acetamidine Hydroiodide is an alkyl halide that can be used to modify the perovskite structure. For example, adjusting the electrical properties of perovskite solar cells and LEDs. And can be used as a reactant in the synthesis of hexagonal polytypes.And can be used as a reactant in the synthesis of hexagonal polytypes.
Acetamidine hydroiodate is needle-like or prismatic crystals. Its melting point is 177~178 °C, and the peak of UV absorption is located at 224nm (soluble in acetamidine). Acetamidine hydroiodate is soluble in water and ethanol, insoluble in acetone, ether and benzene. Its salts react with alkali to produce acetamidine, and its aqueous solution can be decomposed into ammonia and acetic acid when warmed. Therefore, it should be stored at low temperature closure.