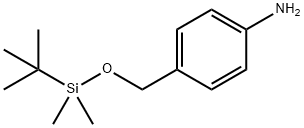
General procedure for the synthesis of 4-((tert-butyldimethylsilyloxy)methyl)aniline from the compound (CAS: 135605-97-9): a mixture of 2a (243 mg, 1 mmol) and 1% Pd/Ni bimetallic catalyst (73 mg, 30 wt%) in methanol (10 mL) was subjected to an atmospheric-pressure hydrogenation unit, and hydrogen was passed through the unit at room temperature and atmospheric pressure. Stirring was continued until the uptake of hydrogen ceased (about 90 min). Upon completion of the reaction, the catalyst was removed by means of a magnetic stirring bar, followed by evaporation of the solvent using a rotary evaporator to afford product 1a as a yellow oil (210 mg, 98% yield), which was pure enough for 1H NMR and 13C NMR analysis. The product was characterized by the following data: IR (KBr) ν 3372, 1623, 1519, 1233 cm-1 ; 1H NMR (CDCl3, 400 MHz) δ 7.26-7.20 (m, 2H), 7.06 (d, 2H, J = 11.0 Hz), 6.87 (d, 2H, J = 11.0 Hz), 6.69 (d, 2H, J = 11.0 Hz), 4.90 (s, 2H), 3.69 (s, 2H), 2.28 (s, 3H); 13C NMR (CDCl3, 100 MHz) δ 156.8, 146.3, 129.7 (2C), 126.8, 115.0 (2C), 114.6 (2C), 70.1, 20.4. HRMS (ESI- TOF) (m/z): calculated value for C14H15NO, [M + Na]+: 236.1046, measured value: 236.1044.
![Benzene, 1-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-4-nitro-](/CAS/20210305/GIF/135605-97-9.gif)