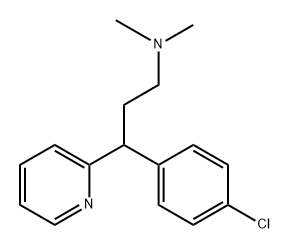
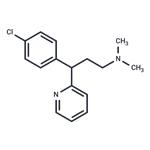
Chlorpheniramine is a drug in the class of first-generation
antihistamines, used to help alleviate symptoms of allergic
reactions potentiated by histamine release. Though it is
included in many multisymptom over-the-counter cold relief
medications, the Food and Drug Administration (FDA) issued
a safety alert in March 2011 detailing some risks associated
with these medications. The safety alert also indicated that
increased enforcement of FDA laws governing the marketing
of these drugs would occur, as many of the products had not
been approved in their current formulations for safety, effectiveness,
and quality.
Chlorpheniramine is commonly used in small-animal
veterinary medicine for its antihistaminic/antipruritic effects,
especially for the treatment of pruritus in cats, and occasionally
as a mild sedative.
ChEBI: Chlorphenamine is a tertiary amino compound that is propylamine which is substituted at position 3 by a pyridin-2-yl group and a p-chlorophenyl group and in which the hydrogens attached to the nitrogen are replaced by methyl groups. A histamine H1 antagonist, it is used to relieve the symptoms of hay fever, rhinitis, urticaria, and asthma. It has a role as a H1-receptor antagonist, an antipruritic drug, a histamine antagonist, a serotonin uptake inhibitor, an antidepressant and an anti-allergic agent. It is a tertiary amino compound, a member of monochlorobenzenes and a member of pyridines.
Toxicity of antihistamines is usually related to their anticholinergic
effects and may include loss of appetite, nausea,
vomiting, diarrhea or constipation, and other GI effects, as well
as dizziness, tinnitus, lassitude, incoordination, fatigue, blurred
vision, diplopia, euphoria, nervousness, insomnia, and
tremors. Acetylcholine is competitively blocked at muscarinic
receptors, resulting in symptoms of anticholinergic poisoning.
Concurrent use of alcohol, tricyclic antidepressants, monoamine
oxidase inhibitors, or other central nervous system
(CNS) depressants along with antihistamines may exaggerate
and extend the anticholinergic and CNS depressant effects of
antihistamines; concurrent use is not recommended.
Products that were marketed prior to the FDA safety alert
but not approved by the FDA included multisymptom cold
medications comprised of drug combinations of chlorpheniramine
with decongestants, antitussives, and analgesics. Risks
associated with the use of these products included improper
use in children and infants, potentially risky combination of
ingredients, and patients receiving too much or too little
medication because of problems with the way some ‘extendedrelease’
products were made. Newborn and premature infants
are even more prone to anticholinergic side effects and an
increased susceptibility toward convulsions; thus, this drug is
not recommended at all in this age group. Geriatric patients are
also more prone to anticholinergic effects, and a paradoxical
reaction characterized by hyperexcitability may occur in some
children taking antihistamines. Overdosage may also produce
central excitation resulting in convulsions.