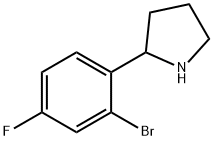
The oily 5-(2-bromo-4-fluorobenzene)-3,4-dihydro-2H-pyrrole (6.05g, 0.025mmoL) was dissolved in 100mL of methanol and water (4:1) solution, cooled to 0 degree,Sodium borohydride (0.95 mg, 0.025 mmol) was added in portions with hydrogen evolution.The reaction solution became a yellow turbid liquid, and after 3 hours, it was allowed to warm to room temperature, and the solvent was distilled off.The residue was treated with KHCO3 and extracted with ethyl acetate.The organic layer was dried over anhydrous sodium sulfate and filtered and evaporated. A pale yellow oil was obtained, and 5.84 g of 2-(2-bromo-4-fluorophenyl)pyrrolidine crude product was obtained, with a yield of 95.7%, which was directly reacted.