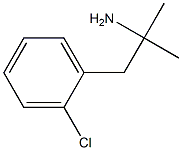
To a Grignard reagent (prepared from 50.0 g of o,α-dichloro-toluene and 7.45
g of magnesium in diethyl ether) is added 18.0 g of acetone at such rate that
constant reflux is maintained. The reaction mixture is allowed to stand
overnight at room temperature, and is then poured onto a mixture of 20%
sulfuric acid and ice. The organic layer is separated, washed with water, an
aqueous solution of sodium hydrogen carbonate and again with water, dried
over magnesium sulfate and evaporated to dryness. The residue is distilled
under reduced pressure to yield 42.6 g of 1-(o-chlorophenyl)-2-methyl-2-
propanol, BP 120° to 122°C/12.5 mm.
To 29.0 ml of glacial acetic acid, cooled to 15°C, is added 11.5 g of sodium
cyanide (98%) while stirring, and then dropwise 32.4 ml of concentrated
sulfuric acid, dissolved in 29 ml of glacial acetic acid, while maintaining a
temperature of 20°C. The 1-(o-chlorophenyl)-2-methyl-2-propanol is added
moderately fast, allowing the temperature to rise spontaneously. After
completing the addition, the reaction mixture is heated to 70°C and stirred,
and is then poured onto a mixture of water and ice. The aqueous mixture is
neutralized with sodium carbonate and extracted with diethyl ether. The
organic solution is washed with water, dried over magnesium sulfate and
evaporated to dryness.
The oily residue is taken up in 100 ml of 6 N aqueous hydrochloric acid and
refluxed until a clear solution is obtained. The latter is made basic with
aqueous ammonia and extracted with diethyl ether; the organic solution is
separated, washed, dried and evaporated. The residue is distilled under
reduced pressure to yield 26.3 g of 1-(o-chlorophenyl)-2-methyl-2-
propylamine, BP 116° to 118°C/16 mm.
The 1-(o-chlorophenyl)-2-methyl-2-propylamine hydrochloride is prepared by
adding ethanolic hydrogen chloride to an ice-cold solution of the free base in
ethanol; the desired salt precipitates and is recrystallized from ethanol, MP
245° to 246°C.