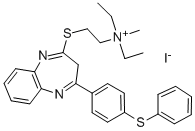
Antoral,Recordati,Italy,1977
4-Acetyldiphenylsulfide is reacted with carbon disulfide in an initial step to
give 4-phenylthiobenzoyl dithioacetic acid. That, in turn, is reacted with o_x0002_phenylenediamine.
A mixture of 3.6 g of the thus obtained 4-p-phenylthiophenyl-1,3-dihydro-2H-
1,5-benzodiazepine-2-thione, 0.50 g of 50% sodium hydride in oil and 200 ml
of benzene is refluxed for 30 minutes, then a solution of 2.02 g of β-
diethylaminoethyl chloride in 5 ml of benzene are added dropwise over 5
minutes.
The mixture is refluxed for 10 hours. The mixture is then cooled and filtered
to separate the sodium chloride. The filtrate is evaporated to dryness in
vacuo. The oily residue is dissolved in petroleum ether and the solution is
filtered with charcoal. The solvent is evaporated in vacuo. The oily residue is
heated to 50°C in vacuo (0.01 mm Hg) to remove the excess of β-
diethylaminoethyl chloride.
This treatment is continued until the β-diethylaminoethyl chloride disappears(TLC). The oil is then dissolved in isopropanol and weakly acidified with HCl in
propanol. The 2β-N-diethylaminoethylthio-4-p-phenylthiophenyl-3H-1,5-
benzodiazepine HCl product crystallizes by addition of anhydrous ethyl ether
to the solution. The crystals are filtered and recrystallized from ethyl acetate.
Yield 3.65 g, melting point 150°C.
2.55 g of methyl iodide are added to a solution of 5.93 g of 2-β-N�diethylaminoethylthio-4-phenylthiophenyl-3H-1,5-benzodiazepine in 100 ml of
isopropanol. The mixture is kept at 20°C to 30°C for 60 hours. The crystals
are then filtered. Yield 6.2 g, melting point 161°C.