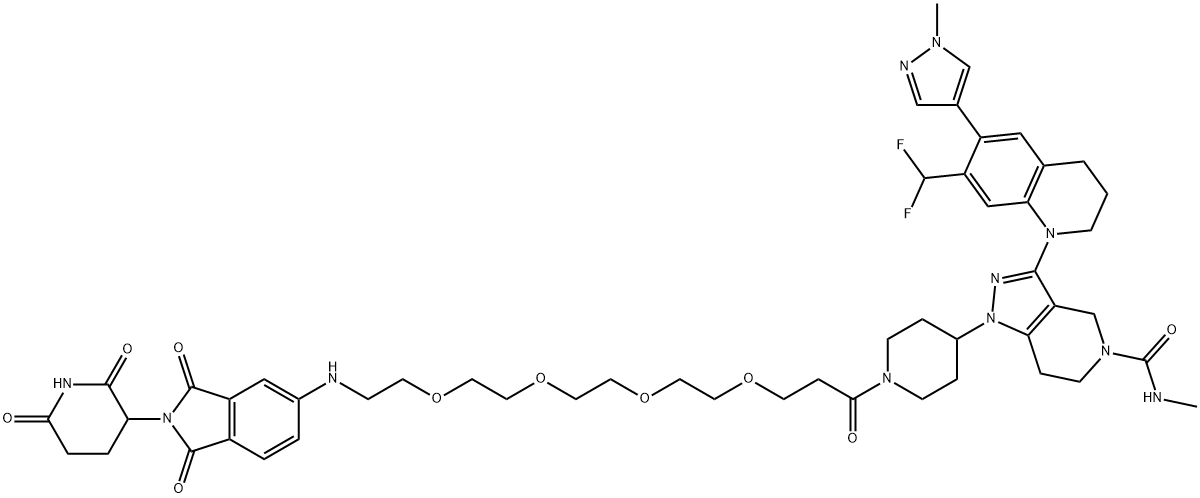
dCBP-1 is exceptionally potent at killing multiple myeloma cells and can abolish the enhancer that drives MYC oncogene expression. As an efficient degrader of this unique class of acetyltransferases, dCBP-1 is a useful tool alongside domain inhibitors for dissecting the mechanism by which these factors coordinate enhancer activity in normal and diseased cells.
dCBP-1 is a potent and selective heterobifunctional degrader of p300/CBP based on PROTAC. dCBP-1 is exceptionally potent at killing multiple myeloma cells and ablates oncogenic enhancer activity driving MYC expression[1].
dCBP-1 (10-1000 nM; 6 hours) treatment shows near-complete degradation of p300/CBP in MM1S cells. dCBP-1 is also able to induce near-complete p300/CBP degradation across other multiple myeloma cell lines tested, including MM1R, KMS-12-BM, and KMS34[1].Treatment of the human haploid cell line HAP1 for 6 h with dCBP-1 reveals almost complete loss of both CBP and p300 between 10 and 1000 nM doses. A time course analysis with 250 nM dCBP-1 revealed almost complete degradation of p300/CBP within an hour of treatment[1].
3-(7-(difluoromethyl)-6-(1-methyl-1H-pyrazol-4-yl)-3,4-dihydroquinolin-1(2H)-yl)-1-(1-(1-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-5-yl)amino)-3,6,9,12-tetraoxapentadecan-15-oyl)piperidin-4-yl)-N-methyl-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxamide dCBP-1: The compound tert-butyl 3-(7-(difluoromethyl)-6-(1-methyl-1H-pyrazol-4-yl)-3,4-dihydroquinolin-1(2H)-yl)-1-(1-(1-((2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindolin-5-yl)amino)-3,6,9,12-tetraoxapentadecan-15-oyl)piperidin-4-yl)-1,4,6,7-tetrahydro-5H-pyrazolo[4,3-c]pyridine-5-carboxylate S18 (0.010 g, 0.01 mmol) was dissolved in 1:1 DCM: TFA (1 mL) and stirred for 30 min. The solvents were evaporated under reduced pressure, and the crude reaction mixture was dissolved in DCM (1 mL), TEA (0.1 mL), and N-methyl-1H-imidazole-1-carboximide (3.5 mg, 0.03 mmol) was added at RT and stirred overnight. Volatiles were evaporated, and the crude reaction mixture was purified by ISCO using C18 (14g) column, 0-100% MeOH/water to yield dCBP-1 (0.008 g, 80%) as a yellow oil.