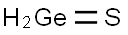Germanium sulfide is a substance that highly absorbs microwave radiation. Therefore, when sulfidation is carried out using microwave radiation, only the sulfides selectively absorb microwave radiation, generating instantaneous high temperatures. This causes germanium to react with sulfur to form germanium sulfide, which continues to absorb microwave radiation until it reaches the temperature for sublimation and volatilization. Microwave radiation has strong and uniform penetrability, resulting in high volatilization efficiency of germanium sulfide without generating excessively high local temperatures. The product obtained is mainly germanium sulfide, which is beneficial for subsequent processing. Compared to fuming furnaces, rotary kilns have superior indirect heating methods and heat conduction through a medium.
reddish-yellow, amorphous or rhomb crystal(s); -20 mesh with 99.95% purity [CER91] [CRC10]
- New flexible and transparent solution-based germanium-sulfide polymeric materials: This research presents the successful preparation of solution-based polymeric germanium sulfide materials, showcasing their potential applications in flexible and transparent electronics (DTB De Salvi, AE Job, SJL Ribeiro, 2015).
- Germanium monosulfide as a natural platform for highly anisotropic THz polaritons: Introduces alpha-germanium(II) sulfide (GeS) as a promising candidate for enhancing THz nanospectroscopy, significantly impacting materials science and optoelectronic device development (T Nörenberg et al., 2022).
Differential thermal analysis of germanium (II) sulfide was investigated. Melting point of GeS is 665oC. GeS nanoparticles may be prepared by gas phase laser photolysis, to be used in lithium ion batteries. GeS films may be generated by electrochemical deposition and used as an electrolyte. A study also reports the formation of GeS clusters by laser ablation.
reagent type: catalyst
core: germanium
(1) A mixture of calcium carbonate with a mass ratio of 2:1 and calcium monomer with a purity level of 99.5% and a mixture of germanium powder, germanium dioxide and germanium monoxide with a purity level of 99.9% with a mass ratio of 4:3:3 were loaded into a quartz tube with a molar ratio of 1:1, discharged to milliTorr pressure on a vacuum sealing machine, sealed under vacuum utilizing a hydroxide flame torch, and annealed at 1000C for 20 hours, cooled to room temperature, the reaction product was stirred in 20 w% HCl solution for 5 days, the temperature was controlled at -20C. After obtaining the product, it was washed with ultrapure water, methanol, and dried in turn to obtain the purified germanium multilayer graphene analog GeH. .
(2) The above product GeH and sulfur powder with a purity level of 99.5% were added to a quartz tube at a molar ratio of 1:5, and sealed under vacuum by using a vacuum tube sealer, and then annealed at 800 C. for 8 hours, and then cooled to room temperature, and then washed with carbon disulfide and ultrapure water in turn, and dried to obtain the layered germanium sulfide.