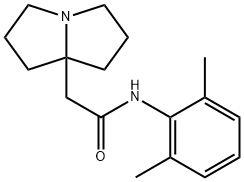
Pilsicainide: C17H24N2OHCl1/2H20. [88069-49-2]. It is obtained from the crystallization of ethanol-diethyl ether. Odorless, bitter taste, melting point 212 ~ 214℃. It is highly soluble in glacial acetic acid and soluble in methanol, ethanol or water.
Pilsicainide is a class IC antiarrhythmic drug, suitable for the treatment of patients with ventricular tachycardia ineffective or intolerable to other antiarrhythmic drugs.
ChEBI: Pilsicainide is a secondary carboxamide resulting from the formal condensation of the amino group of 2,6-dimethylaniline with the carboxy group of (tetrahydro-1H-pyrrolizin-7a(5H)-yl)acetic acid. It is a sodium channel blocker which is used as an antiarrhythmic drug for the management of atrial tachyarrhythmias in Japan. It has a role as an anti-arrhythmia drug and a sodium channel blocker. It is a secondary carboxamide and an organic heterobicyclic compound.
Pilsicainide was obtained from tetrahydrodithickened pyrrole ethyl acetate and 2, 6-dimethylaniline heated and condensed in dioxane at 100℃ under the action of sodium hydride. It is also possible to hydrolyze tetrahydrodithickened pyrrole ethyl acetate and chlorinate it to tetrahydrodithickened pyrrole acetyl chloride, which is then condensed with 2, 6-dimethylaniline.
Pilsicainide (SUN 1165 free acid; 2 mg/kg; i.v.; once) decreases the conduction velocity in T4-treated rat atrium by decreasing the Max dV/dt and net inward current[1].
| Animal Model: | Male Sprague-Dawley (SD) rats weighing from 200 to 220 g, with levo-thyroxine (T4) treatment[1] |
| Dosage: | 2 mg/kg |
| Administration: | Bolus injection into right external carotid vein within 2 minutes, once |
| Result: | Elongated the QT interval at 15 and 60 minutes after administration.
Shortened P wave and QRS complex durations.
Markedly decreased action potential amplitudes (APA) and Max dV/dt, and significantly lengthened the action potential durations.
|