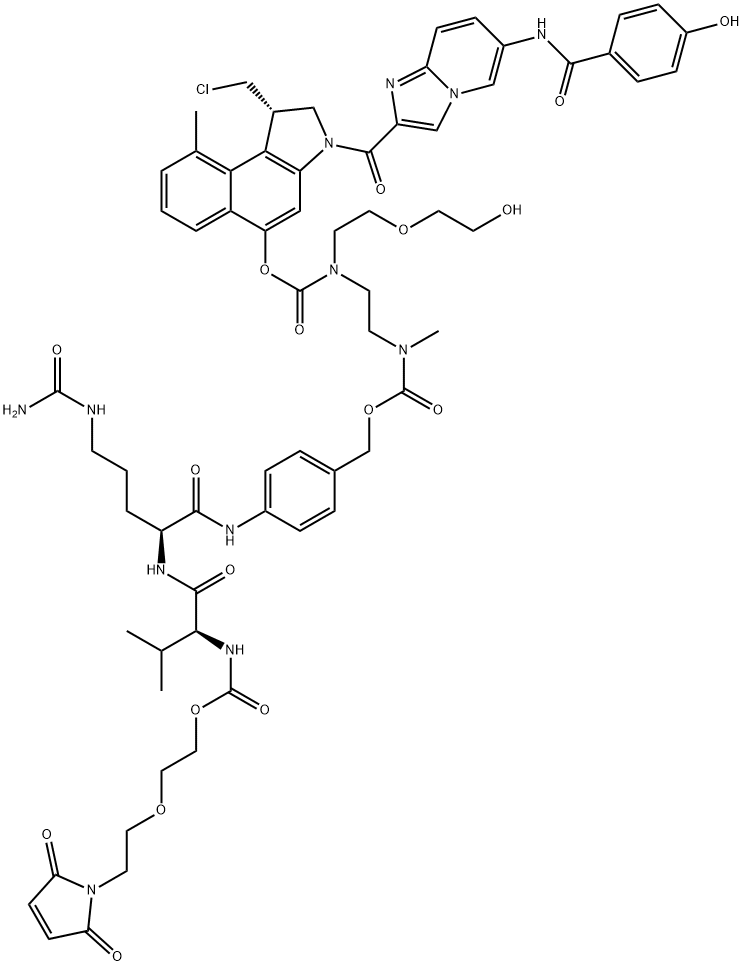
Vc-seco-DUBA is a novel HER2-targeting antibody–drug conjugate used in the treatment of cancer. Trastuzumab duocarmycin, also known as SYD985, is a new HER2-targeted ADC with a cleavable payload (vc-seco-DUBA) conjugated with trastuzumab[1].
Vc-seco-DUBA, a cleavable linker-duocarmycin payload, could used to synthsis SYD985 with trastuzumab.
Vc-seco-DUBA is Danger.
H340 (100%): May cause genetic defects [Danger Germ cell mutagenicity]
H351 (100%): Suspected of causing cancer [Warning Carcinogenicity]
H373 (100%): Causes damage to organs through prolonged or repeated exposure [Warning Specific target organ toxicity, repeated exposure]
SYD985 ( based on trastuzumab and Vc-seco-DUBA) and T-DM1 induced similar ADCC in the presence of peripheral blood lymphocytes (PBL) against EOC cell lines with differential HER2/neu expression. In contrast, SYD985 was 3 to 42 fold more cytotoxic in the absence of PBL when compared to T-DM1 (p<0.0001). Unlike T-DM1, SYD985 induced efficient bystander killing of HER2/neu 0/1+ tumor cells when admixed with HER2/neu 3+ EOC cells. In vivo studies confirmed that SYD985 is significantly more active than T-DM1 against HER2/neu 3+ EOC xenografts[2].
[1] Ubink R, et al. Unraveling the interaction between carboxylesterase 1c and the antibody-drug conjugate SYD985: improved translational PKPD by using CES1c knockout mice. Molecular Cancer Therapeutics, 2018; 17: 2389–2398.
[2] Menderes G, et al. SYD985, a novel duocarmycin-based HER2-targeting antibody-drug conjugate, shows promising antitumor activity in epithelial ovarian carcinoma with HER2/Neu expression. Gynecologic Oncology, 2017; 146: 179-186.