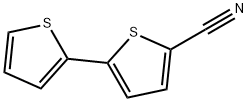
5-Cyano-2,20-bithiophene can react with hydrazine to synthesize five-ring compounds. It can be converted to dinitriles by Heck coupling reaction and synthesized phenyl-2,2-bichalcophene diamidines from dinitriles. These diamines have a wide DNA affinity and are very sensitive to replacing furan units with thiophenes. It can also be used as an intermediate in the synthesis of 3,6-bis[5-(2,2-bithiophene)]pyrrolo[3,4-c]pyrrole-1,4(2H,5H)-dione for the preparation of organic electronic devices.