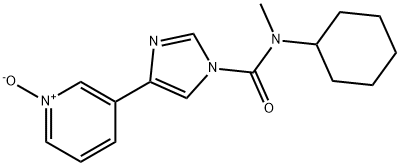
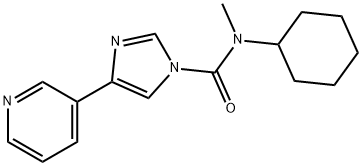
In a further step of this synthesis, N-cyclohexyl-N-methyl-4-(pyridin-3-yl)-1H-imidazole-1-carboxamide (90 g, 317 mmol) was dissolved in dichloromethane (1350 ml) in a 2L reactor followed by addition of peracetic acid (84 ml, 475 mmol). The reaction mixture was stirred at 25 °C and the progress of the reaction was monitored by HPLC until N-cyclohexyl-N-methyl-4-(pyridin-3-yl)-1H-imidazole-1-carboxamide was completely consumed. After completion of the reaction, an aqueous solution (270 ml) of sodium metabisulfite (60.2 g, 317 mmol) was slowly added, keeping the reaction temperature below 30 °C. After performing phase separation, the organic phase was washed with water. After phase separation again, the organic phase was concentrated to 1/5 of the original volume at atmospheric pressure.Subsequently, the solvent was replaced with isopropanol (1350 ml) and the suspension was cooled down to 0 °C over a period of 4 hours and stirring was continued at this temperature for 1 hour. The resulting white crystalline solid was collected by filtration and washed sequentially with water (270 ml) and isopropanol (270 ml) to give 84.8 g (89% yield) of the target product.