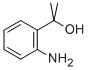
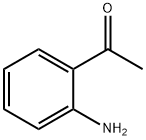
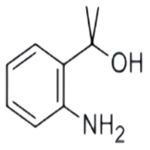
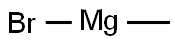GENERAL STEPS: 1-(2-aminophenyl)ethanone (1.50 g, 11.1 mmol) was dissolved in tetrahydrofuran (11 mL) under argon protection and cooled at 0 °C. Subsequently, methylmagnesium bromide (1.0 mol/L tetrahydrofuran solution, 11.1 mL, 11.1 mmol) was added slowly. After addition, the reaction mixture was gradually warmed to room temperature and stirred. Upon completion of the reaction, the reaction was carefully quenched with saturated aqueous ammonium chloride solution. The reaction mixture was extracted with ethyl acetate (3 x 20 mL), the organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The crude product obtained was a brown oil, which was purified by fast column chromatography to give 2-(2-aminophenyl)propan-2-ol as a light-colored oil (1.20 g, 72% yield).LC-MS analysis showed a retention time of 0.24 min, and m/z 134 ([M-OH]-) was observed in ESI+ mode.1H NMR (400 MHz, chloroform-d) δ ( ppm): 7.15 (d, 1H), 7.06 (t, 1H), 6.72 (t, 1H), 6.65 (d, 1H), 3.65 (br s, 1H), 1.67 (s, 6H).