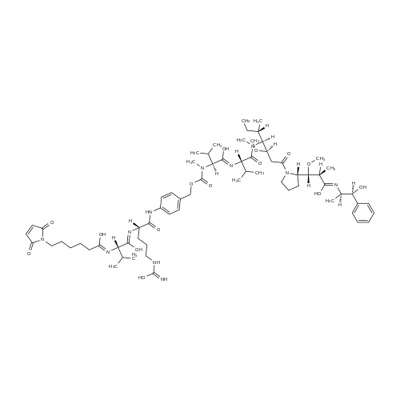
MC-Val-Cit-PAB-MMAE is a precursor of antibody drug conjugate. It contains a thio reactive maleimidocaproyl (MC) group, a protease-sensitive Val-Cit dipeptide, a PABC linker and a MMAE payload. The MMAE is a synthetic antineoplastic agent. It can be attached to a monoclonal antibody (MAB) which directs it toward cancer cells.
Vedotin is an ultra-high-affinity small organic ligand of fibroblast activation protein used for tumor-targeting applications
VcMMAE is a MMAE derivative with valine-citrulline (Vc) linker. VcMMAE can be used to make antibody drug conjugate. VcMMAE is a anti-mitotic agent, monomethyl auristatin E (MMAE), linked via the lysosomally cleavable dipeptide, valine-citrulline (vc). Monomethyl auristatin E (MMAE) is a synthetic antineoplastic agent. Because of its toxicity, it cannot be used as a drug itself; instead, it is linked to a monoclonal antibody (MAB) which directs it to the cancer cells. In International Nonproprietary Names for MMAE-MAB-conjugates, the name vedotin refers to MMAE plus its linking structure to the antibody. It is a potent antimitotic drug derived from peptides occurring in marine shell-less mollusc Dolabella auricularia called dolastatins which show potent activity in preclinical studies, both in vitro and in vivo, against a range of lymphomas, leukemia and solid tumors. These drugs show potency of up to 200 times that of vinblastine, another antimitotic drug used for Hodgkin lymphoma as well as other types of cancer?
VcMMAE is an antibody-drug conjugate (ADC) with potent antitumor activity by using the anti-mitotic agent, monomethyl auristatin E (MMAE), linked via the lysosomally cleavable dipeptide, valine-citrulline (vc). As a monoclonal antibody, it acts by binding to the extracellular domain of epidermal growth factor receptor (EGFR) and blocking its interaction with the ligands, thereby inhibiting cellular proliferation.
MMAE is efficiently released from SGN-35 within CD30 cancer cells and, due to its membrane permeability, is able to exert cytotoxic activity on bystander cells+. MMAE sensitized colorectal and pancreatic cancer cells to IR in a schedule and dose dependent manner correlating with mitotic arrest. Radiosensitization is evidenced by decreased clonogenic survival and increased DNA double strand breaks in irradiated cells.
MMAE in combination with IR results in tumor growth delay, tumor-targeted ACPP-cRGD-MMAE with IR produces a more robust and significantly prolonged tumor regression in xenograft models.